• Physics 16, 114
A concept in condensed matter physics called jamming provides a possible prognostic tool for cancer.
Cancer is a very complex and multifaceted disease accounting for nearly one in six deaths worldwide. More than 90 percent of cancer deaths are due to metastasis, the process by which cancer cells escape the primary tumor, spread through the body, and seed a secondary tumor in a distant organ or tissue. Given these sobering numbers, it should come as no surprise that researchers consider a better mechanistic understanding of metastasis of paramount importance in the fight against cancer. What is perhaps most surprising is that a key role in this struggle can be played by the physics of condensed matter. Pablo Gottheil of the University of Leipzig in Germany and colleagues illustrated why this is the case by showing that concepts of soft condensed matter – in particular, the physics of disordered systems – can improve long-term metastatic risk assessment of cancer patients breast [1].
Physicists have long contributed to cancer research by developing, for example, advanced imaging tools. But it’s only in the last decade or so that the development of the disease itself, particularly the occurrence of metastases, has been recognized as a real physics problem. The basic idea is simple: No matter how complex the genetic and biochemical signatures of cancer, all cancer cells must ultimately obey the laws of physics as they move, deform and migrate through the body. In recent years, this uniquely physics approach, dubbed the physics of cancer, has emerged as a promising paradigm for providing insights into key aspects of cancer progression.
Conventionally, the physics of disordered systems deals with the behavior of inanimate materials such as granules, complex fluids and glasses. A special property of these materials is that they can rapidly switch from liquid to solid behavior while preserving their disordered structure, an intriguing phenomenon known as jamming. [2]. About a decade ago, pioneering work established that living cells also exploit physical jamming in densely packed cell layers and tissues [3]. Indeed, just as a pile of sand can slip through your fingers one moment and be part of a solid sand castle the next, deformable cells can go from mobile to stationary state without compromising the structural integrity of the tissue. Even more compelling is the growing awareness that such cellular locking and unlocking transitions could have important biological functions in both health and disease. [4, 5].
The importance of unblocking in cancer is rooted in a simple physics notion: In order for cancer cells to metastasize, they have to move. The enhanced cell motility in cancer tissue can be triggered by a multitude of signals, including genetic and environmental signals [6]. However, mounting evidence suggests that a disorder-free emergent transition is at play even in primary tumors. But how can cells unblock in a compact solid tumor and to what extent does unblocking really matter for the final metastatic outcome? Both of these questions have now been addressed by Gottheil and colleagues in a groundbreaking study that combines physics-based principles with clinical data from cancer patients.
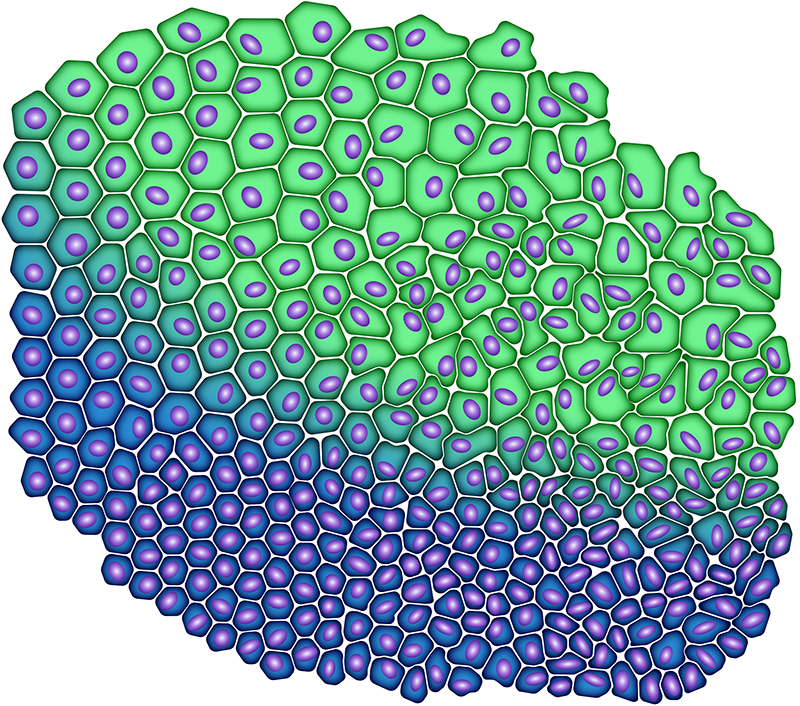
To answer the first question, the researchers build on the last decade’s discovery that cell shape can be a very good predictor of the degree of jamming in dense cell layers. [7, 8]. Explicitly, elongated cells tend to be more mobile than round ones because they can more easily slip between and swap positions with their neighbors. But cell shape alone is not a sufficient structural order parameter for jamming and unblocking in solid tumors [1, 9]. Gottheil and colleagues use patient-derived breast cancer cells to show that the nucleus of the cell should also be considered. One physically intuitive reasoning for this discovery is that the nucleus is a relatively large and rigid object that can mechanically impede a cell’s ability to move through a dense environment.
The researchers combine knowledge of cell and nuclear shapes in a new order parameter called CeNuS. They therefore propose a link between this simple static information and emerging cell motility – and thus the degree of disinhibition – in primary breast cancer cells. This morphodynamic linkage also forms the basis for a tumor cell undocking status plot (Fig. 1), which can be used to readily estimate the undocking status for a given tissue sample from a patient.
To explore whether a greater degree of unblocking is also related to a higher metastatic risk, Gottheil and colleagues take another important step by performing a retrospective study of clinical data from 1380 breast cancer patients. Surprisingly, the researchers find that an increase in expected unblocking significantly correlates with the development of distant metastases that could occur even a decade later. This finding also implies that cell unblocked status, as assessed by CeNuS, may serve as a complementary prognostic biomarker for cancer. Indeed, when combined with conventional risk assessment criteria, the inclusion of physical detoxification increases the accuracy of prognosis for metastases from 66.8% to 71.3%. This improvement corresponds to a 26% increase in prognostic information compared to using established parameters alone. Collectively, these results suggest that the emerging physics of disordered condensed matter could play a vital role in metastasis.
Aside from the relevance to cancer, we recall that, more generally, jamming and related glass phenomena are based on subtle structural changes that act to liquefy or solidify an amorphous material. On the one hand, the absence of major structural changes makes understanding these transitions one of the most complex problems in classical physics. But on the other hand, it offers a wonderful practical convenience [10]: With only minimal particle rearrangements or deformations, a system can easily alter its collective properties. It is plausible that living cells and tissues have cleverly exploited this behavior by evolving to a state that remains close to a jamming or unlocking threshold, such that they can efficiently transition from highly motile to dormant. In the coming years, we can therefore expect the physics of disordered systems to gain more and more recognition in the biological sciences.
References
- P. Gottheil et al.Cell unblocking status correlates with distant metastases in cancer patients, Phys. Reverend X 13031003 (2023).
- AJ Liu and SR Nagel, Jamming is not just cool anymore, Nature 39621 (1998).
- M. Sadati et al.Collective migration and cell jamming, Differentiation 86121 (2013).
- L. Atia et al.Are jamming and unblocking of cells essential for tissue development? Dev. Cells 168203727 (2021).
- E.Blauth et al.Jamming in embryogenesis and cancer progression, In front of. Phys. 9666709 (2021).
- J. A. Mitchell et al.In primary airway epithelial cells, the uninhibited transition is distinct from the epithelial-to-mesenchymal transition, Nat. Common. 115053 (2020).
- J.-A. Park et al.Cell detachment and shape in asthmatic airway epithelium, Nat. Mother. 141040 (2015).
- D.Bi et al.Motility-driven glass and disturbance transitions in biological tissues, Phys. Reverend X 6021011 (2016).
- S. Grosser et al.Cell shape and nucleus as an indicator of tissue fluidity in carcinoma, Phys. Reverend X 11011033 (2021).
- LMC Janssen, Active glasses, J. Fis. Condensation. Question 31503002 (2019).
About the author
Thematic areas
#physics #cancer #takes #shape
Image Source : physics.aps.org