Researchers give an update on clinical trials with CB2R agonists and their potential for the treatment of inflammatory diseases
Activation of cannabinoid receptor type 2 (CB2R) has great potential for the treatment of diseases with an inflammatory component. This G protein-coupled receptor (GPCR) is a key element of the endocannabinoid system (ECS), an important lipid signaling system.
Numerous small-molecule activators, so-called CBs2R-agonists have demonstrated high efficacy in preclinical disease models for the treatment of a multitude of pathological conditions such as cardiovascular, gastrointestinal, hepatic, renal, pulmonary, neurodegenerative/neuroinflammatory, cutaneous, rheumatoid arthritis, endometriosis, and eye diseases . (1)
Furthermore, many of these CB2R-agonists have progressed to clinical stages and may soon provide solutions for unmet medical needs and inflammatory diseases.
From Cannabis sativa to selective CB2R agonists
Since at least 1000 BC, Cannabis sativa has been used for recreational and medicinal purposes, including anti-inflammatory treatments. However, it wasn’t until 1964 that its main active ingredient, 9-tetrahydrocannabinol (THC), was identified.(2)
The discovery of its drug targets, cannabinoid receptors type 1 and type 2 (CB1R and CB2R) did not occur until 1988(3) and 1993 (4), respectively. THC is a potent double CB1R and CB2R agonist exhibiting multiple therapeutically relevant physiological properties, including anti-inflammatory, immunosuppressive, and analgesic effects. However, Central CB1R-mediated psychotropic outcomes have led to regulation of its use.(5)
To overcome this, two drug development strategies have been followed by pharmaceutical chemists: i) Limit drug exposure towards the periphery so as not to interact with the brain CB1R; and ii) improve the selectivity on CB1R.
Clinical trials with CB2R agonists
Overall, more than 20 CB2R agonists have been studied in humans for a wide range of indications. The chemical structures of drugs in this context are very diverse.
Fatty acid derivatives, classical and non-classical cannabinoids, and multiple different synthetic ligands are included, which also results in the coverage of a wide range of physicochemical properties. (1c, 6)
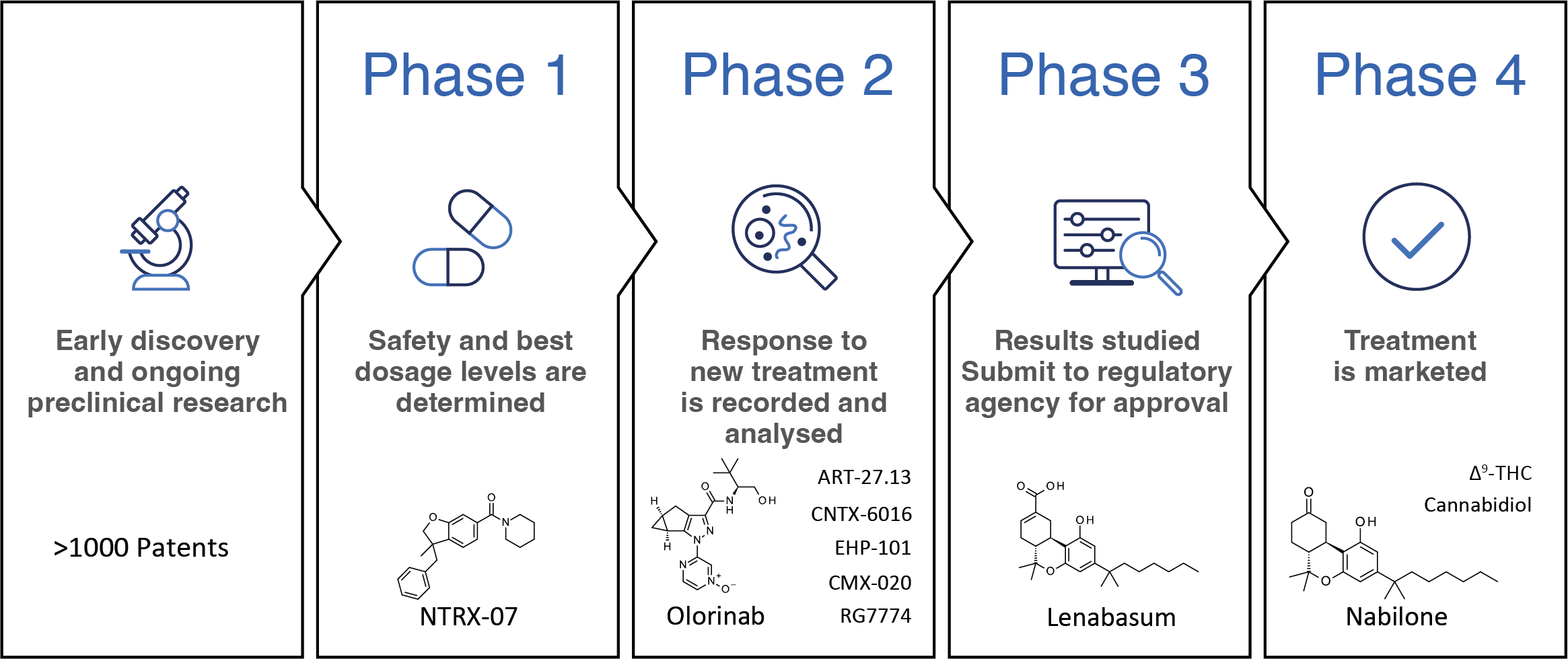
In clinical studies, data on dosage, safety and therapeutic efficacy in humans are generated (Figure 1).
The overall design of the study aims to ensure the scientific validity and reproducibility of the results. Generally, clinical trials are divided into four phases.(7)
In Phase 1, information about the pharmacokinetic profile of a new molecular entity is collected in a small group of people.
In addition, safe dosage regimens and side effect profiles are determined. Phase 2 focuses on establishing the preliminary efficacy of the drug in patients, usually against a placebo control group.
Phase 3 aims at definitive confirmation of safety and efficacy data and is followed by the launch of the medicine. Finally, Phase 4 trials continuously monitor a drug and outline its risks, benefits, and optimal use over its lifetime.
CB launched2R-agonists and ongoing clinical trials
The first mostly non-selective CB2R-agonists, which often had unfavorable absorption, distribution, metabolism, and excretion (ADME) profiles and often focused on pain indications, were discontinued in clinics for various reasons, but did not increase CB2R related security issues.(8)
Phytocannabinoids Dronabinol, i.e. synthetic THC, Nabilone and Cannabidiol (CBD) which exert their action partially through the CB2R activation, have been introduced to the market. Oral THC is used to treat anorexia, cachexia, and chemotherapy-induced emesis.
At the same time, buccal THC was launched for cancer pain. Nabilone is used to treat patients suffering from chemotherapy induced nausea and vomiting. CBD, a non-classical cannabinoid for which the primary mode of action is still debatable, is marketed for the treatment of childhood severe myoclonic epilepsy, Dravet and Lennox-Gastaut syndrome, and tuberous sclerosis.
Combinations of CBD and THC have been approved for the treatment of MS-associated spasticity and pain management. Non-psychoactive Dual CB1R/CB2The R agonist Lenabasum is currently in Phase 3 trials for the treatment of systemic sclerosis and dermatomyositis.
The most advanced selective CB2The R agonists are the synthetic cannabinoids Olorinab and RG7774. The clinical focus of Olorinab is on pain related to irritable bowel syndrome (IBS) and IBS with predominant constipation or diarrhea. RG7774 aims to provide oral treatment to patients suffering from diabetic retinopathy. Arachidonic acid analog CMX-020 is being studied in Phase 2 studies for the treatment of pain, osteoarthritis and diabetic neuropathy.
Pain, particularly neuropathic pain, is also a focus of selective synthetic SCO2R agonists CNTX-6016 (phase 2) and NTRX-07 (phase 1). Double CB1R/CB2R agonist ART-27.13 is in phase 2, seeking to provide treatment options for cancer-related anorexia and cachexia. EHP-101 cannabidiol derivative, which is activated in addition to CB2R also PPAR, is aimed at patient populations affected by multiple sclerosis and scleroderma (phase 2).
Together, these CB2R-agonists have a very high potential for the treatment of multiple diseases in which inflammatory processes play a significant role or are the underlying reasons.
Furthermore, recent progress in expanding the knowledge of CB2The R protein structure and molecular pharmacology will enable the development of the next generation of CB2R agonist drugs, allowing for even more precise drug targeting, thus unlocking the high therapeutic potential of CB2R for the treatment of inflammatory diseases.
Thanks
We thank all colleagues who have contributed to our investigation of CB2R over the years.
References
- a) Maccarrone M, Grether U (2022) Is CB2R a hidden treasure for the treatment of inflammatory diseases? Open Access Government, https://www.openaccessgovernment.org/article/cb%e2%82%82r-treating-inflammatory-diseases-endocannabinoid-system/145866/; b) Pacher P, Grether U (2023) Challenges leading CB2Medicine R at the bedside. Open Access Government, https://www.openaccessgovernment.org/article/challenges-bringing-cb%E2%82%82r-medicine-to-bedside/150463/; c) Maccarrone M, Di Marzo V, Gertsch J et al (2023) Good and bad of the endocannabinoid system as a therapeutic target: lessons learned after 30 years. Pharmacol Rev. Epub before print, doi:10.1124/pharmrev.122.000600.
- Gaoni Y, Mechoulam R (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:16461647.
- Devane WA, Dysarz FA, Johnson MR et al (1988), Determination and characterization of a cannabinoid receptor in the rat brain. Mol. Pharmacol. 34(5):605-613 .
- Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral cannabinoid receptor. Nature 365: 61-65.
- Robson P (2001), Therapeutic aspects of cannabis and cannabinoids. Fr. J. Psychiatry 178:107-115.
- Whiting ZM, Yin J, de la Harpe SM et al (2022) Development of the cannabinoid receptor 2 (CB2) pharmacopoeia: past, present and future. Trends Pharmacol Sci. 43(9):754-771.
- US Food and Drug Administration (2018) What are the different types of clinical research? https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research. Retrieved May 25, 2023.
- Brennecke B, Gazzi T, Atz K et al (2021) Cannabinoid receptor type 2 ligands: an analysis of patents granted since 2010. Pharm Pat Anal 10:111-163.
Note: This is a commercial profile
#CB2R #agonists #clinics #treasure #chest #treating #inflammatory #diseases
Image Source : www.openaccessgovernment.org
